|
|
|
|
 News Feeds News Feeds
|
 Join us on Facebook Join us on Facebook
|
 Follow us on Twitter Follow us on Twitter
|
|
? Posted: Jun 29th, 2012 Posted: Jun 29th, 2012 Posted: Jun 29th, 2012 Posted: Jun 29th, 2012 Posted: Jun 29th, 2012 Posted: Jun 28th, 2012 Posted: Jun 28th, 2012 Posted: Jun 28th, 2012 Posted: Jun 28th, 2012 Posted: Jun 28th, 2012 Posted: Jun 28th, 2012 Posted: Jun 28th, 2012 Posted: Jun 28th, 2012 Posted: Jun 28th, 2012 Posted: Jun 28th, 2012 Posted: Jun 28th, 2012 Posted: Jun 28th, 2012 Posted: Jun 28th, 2012 Posted: Jun 28th, 2012 Posted: Jun 27th, 2012 Posted: Jun 27th, 2012 Posted: Jun 27th, 2012 |
| Posted: Jun 28th, 2012 | |
| New lithium ion battery strategy offers more energy, longer life cycle | |
| (Nanowerk News) Lithium ion batteries drive devices from electric cars to smartphones. And society is demanding more batteries with more capacity from each battery. | |
| To help meet this demand, Environmental Molecular Sciences Laboratory users and researchers put their energy behind a clever new idea that, literally, gives batteries a bit of room to grow (see paper in Nano Letters: "A Yolk-Shell Design for Stabilized and Scalable Li-Ion Battery Alloy Anodes"). | |
| Lithium ion batteries generate electricity by shuttling lithium ions through an electrolyte. In a fully charged battery, lithium ions are stored in a cathode, such as lithium cobalt oxide (LiCoO2). When in use, lithium ions flow from the cathode through an electrolyte into the anode, most commonly made of carbon. During recharging, the ions are pushed back to the cathode where they started. | |
 |
|
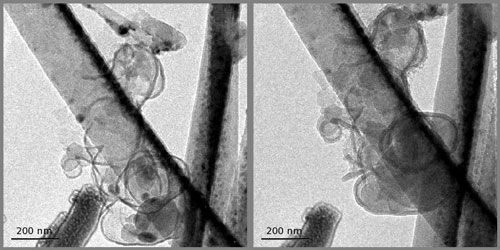
| In situ transmission electron microscopy at EMSL was used to study structural changes in the team?s new anode system. Real-time measurements show silicon nanoparticles inside carbon shells before (left) and after (right) lithiation. | |
| Researchers built upon current technology by making a new type of anode that consists of single silicon nanoparticles inside carbon shells, much like yolks inside eggs. In this new design, lithium ions flow from the cathode through the electrolyte, diffuse through the carbon shells, and enter the silicon?which can hold ten times as many lithium ions as carbon alone. By leaving just the right amount of space, the lithiated silicon nanoparticles swell to fill, but not burst, the carbon shell. | |
| The result? A lithium ion battery system that compared to commercial batteries holds seven times more energy and can be discharged and recharged five times as many times before it wears out. Critical to its good performance, the new system forms a stable crust, a solid electrolyte interphase, on the anode that is a consequence of electrolyte decomposition. Moreover, the team?s manufacturing process is affordable, efficient, and can be readily scaled up. |
?
?
Source: http://www.nanowerk.com/news/newsid=25747.php
hope solo texas high school football fugazi fugazi indiana jones and the last crusade nba lockout dramamine
No comments:
Post a Comment